Chiral photoswitches based on helical oligoarylenes
Chiroptical photoswitches are of particular interest in terms of advanced informational technologies.
Circularly polarized luminescence (CPL) has been attracting much interest as an important chiroptical phenomenon which offers potential applications in sensors, display and optical storage. Meanwhile, the chiroptical photoswitches have often been demonstrated by the stereoselective isomerization of photochromic molecules, changing circular dichroism (CD) and optical rotatory dispersion (ORD).
However, dynamic photoswitching of CPL in solution with a unimolecular system has still remained one of the important challenges in the development of chiroptical photoswitches.
Here we report a chiroptical photoswitch which dynamically modulates CPL emission using a photochromic tetrathiazole scaffold.[1]
In the previous work, we reported that the tetrathiazole folded into a one-turn helical conformation with high photochromic activity by multiple intramolecular interactions.[2]
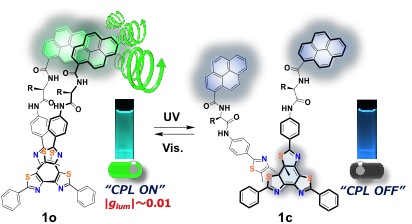
The side-phenyl groups attached to the 2-position of both end-thiazole units were found close to each other with p-p stacking. Pyrene fluorophores are introduced in these positions via a spacer of phenylalanine (1o, Fig. 1).
The chiral spacer is expected to control the handedness of helix, arranging the pyrene-stack in a chiral manner. Chiral excimers of pyrenes have been reported to exhibit relatively strong CPL with |glum| value of 1%, which is given by the equation glum = 2(IL − IR)/(IL + IR), where IL and IR are the intensities of the left- and right-handed circularly polarized emissions, respectively.
Unlike conventional diarylethenes, the photochromism in a tetrathiazole gives rise to a large structural change from a photoreactive conformation to a ring-closed photoisomer, controlling the chiral stack of pyrenes in a dynamic manner (Fig. 1).[3]
[1] Gavrel, G.; Yu, P.; Léaustic, A.; Guillot, R.; Métivier, R.; Nakatani, K. Chem. Commun. 2012, 48, 10111.
[2] Nakashima, T.; Yamamoto, K.; Kimura, Y.; Kawai, T. Chem. Eur. J. 2013, 19, 16972.
[3] Hashimoto, Y.; Nakashima, T.; Shimizu, D.; Kawai, T. Chem. Commun. 2016, 52, 5171.